Where to Buy Piroxicam 7 Mg Tablets for My Dog
 | |
 | |
| Clinical information | |
|---|---|
| Deal out name calling | Mobic, Metacam, Muvera, Anjeso, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601242 |
| License data |
|
| Gestation category |
|
| Routes of administration | By mouth, intravenous |
| ATC code |
|
| Lawful status | |
| Collection status |
|
| Pharmacokinetic data | |
| Bioavailability | 89%[4] |
| Protein binding | 99.4%[4] |
| Metabolism | Liver (CYP2C9 and 3A4-mediated)[4] |
| Elimination incomplete-life history | 20 hours[4] |
| Excretion | Piddle and faeces equally[4] |
| Identifiers | |
| IUPAC name
| |
| CAS Number |
|
| PubChem CID |
|
| IUPHAR/BPS |
|
| DrugBank |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| ChEBI |
|
| ChEMBL |
|
| PDB ligand |
|
| CompTox Dashboard (EPA) |
|
| ECHA InfoCard | 100.113.257 |
| Chemical and physical information | |
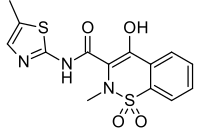
| Formula | C 14 H 13 N 3 O 4 S 2 |
| Molar mass | 351.40 g·mol−1 |
| 3D model (JSmol) |
|
| SMILES
| |
| InChI
| |
| | |
Meloxicam, sold under the brand name Mobic among others, is a nonsteroidal anti-inflammatory (NSAID) wont to treat ail and inflammation in creaky diseases and osteoarthritis.[5] [6] It is used by mouth or by injection into a vein.[6] [7] It is recommended that it be used for as short a flow as possible and at a underslung dose.[6]
Green side personal effects include abdominal pain, lightheadedness, swelling, headache, and a rash.[6] Serious side effects may include heart disease, stroke, kidney problems, and stomach ulcers.[6] Use is not recommended in the third trimester of maternity.[6] It blocks Cox-2 (COX-2) more than it blocks cyclooxygenase-1 (Coxswain-1).[6] It is in the oxicam family of chemicals and is tight concomitant piroxicam.[6]
Meloxicam was patented in 1977 and authorised for medical use in the Coalescing States in 2000.[6] [8] It was developed by Boehringer Ingelheim; however, it is also available as a generic medication.[6] In 2019, it was the 31st most ordinarily prescribed medication in the United States, with more than 21million prescriptions.[9] [10] An intravenous version of meloxicam (Anjeso) was approved for medical use in the United States in February 2020.[7]
Adverse personal effects [edit]
Meloxicam use can solution in gastrointestinal toxicity and bleeding, headaches, rash, and very dark or black stool (a sign of enteral bleeding). Like other NSAIDs, its use is associated with an hyperbolic risk of vas events such equally heart attack and stroke.[11] It has fewer gastrointestinal pull effects than diclofenac,[12] Feldene,[13] Naprosyn,[14] and perhaps all other NSAIDs which are not COX-2 selective.[12] Although meloxicam inhibits formation of thromboxane A, it does not appear to do sol at levels that would step in with platelet operate.[15] [16]
A pooled depth psychology of randomized, controlled studies of meloxicam therapy of equal to 60 days duration found that meloxicam was associated with a statistically significantly lower number of thromboembolic complications than the NSAID diclofenac (0.2% versus 0.8% severally) only a similar incidence of thromboembolic events to naproxen and piroxicam.[17]
In October 2020, the U.S. Food and Drug Administration (FDA) required the do drugs label to be updated for all nonsteroidal anti-inflammatory medications to describe the risk of exposure of kidney problems in unhatched babies that result in low amniotic liquid.[18] [19] They commend avoiding NSAIDs in with child women at 20 weeks or later in pregnancy.[18] [19]
Vas [delete]
People with high blood pressure, high cholesterol, or diabetes are at risk for cardiovascular side personal effects. People with family account of cardiopathy, heart attack, or cam stroke should order their treating physician as the potential for important cardiovascular side effects is significant.[20] [21]
Gastrointestinal [edit]
NSAIDs cause and increase the risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the tummy or intestines, which can be fatal. Senior patients are at greater risk for serious GI events.[22]
Mouth [edit]
It is recommended to withhold meloxicam use for leastwise quaternion to six uncomplete-lives prior to surgical or dental procedures due to increased risk for taste perversion, ulcerative stomatitis and dry mouth.[ medical citation needed ]
Mechanism of action [edit]
Meloxicam blocks cyclooxygenase (Cyclooxygenase), the enzyme answerable for converting arachidonic acid into prostaglandin H2—the first stone's throw in the synthesis of prostaglandins, which are mediators of inflammation. Meloxicam has been shown, especially at low therapeutic doses, to selectively inhibit COX-2 over COX-1.[4]
Meloxicam concentrations in synovial fluent range from 40% to 50% of those in plasma. The free fraction in synovial fluid is 2.5 multiplication high than in plasma, due to the get down albumin content in synovial fluid as compared to plasma. The significance of this penetration is unknown,[22] but it may calculate for the fact that it performs exceptionally well in handling of arthritis in animal models.[23]
Pharmacokinetics [edit]
Absorption [edit]
The bioavailability of meloxicam is decreased when administered orally compared to an equivalent IV bolus dose. Use of oral meloxicam following a high-fat breakfast increases the mean peak do drugs levels away about 22%; however, the manufacturer does non make any specific meal recommendations. In addition, the use of antacids does not show pharmacokinetic interactions.[2]
Statistical distribution [edit out]
The mean volume of statistical distribution of meloxicam is approximately 10L. It is highly protein-bound, mainly to albumin.[16] [24]
Metabolism [cut]
Meloxicam is extensively metabolized in the liver by the enzymes CYP2C9 and CYP3A4 (minor) into tetrad inactive metabolites. Peroxidase activity is thought to live responsible for the other two remaining metabolites.[2] [25]
Excretion [edit]
Meloxicam is predominantly excreted in the form of metabolites and occurs to equal extents in the urine and feces.[2] Traces of unchanged raise drug are found in urine and feces.[2] The mean elimination half-life ranges from 15 to 20 hours.[2]
Specific populations [delete]
Gerontology [edit]
Use of meloxicam is not recommended in citizenry with peptic ulcer disease or accumulated gastrointestinal phlebotomise lay on the line, including those over 75 years old or those winning medications associated with bleeding risk.[26] Adverse events have been found to be dose-dependent and associated with duration of discussion.[26] [2]
Veterinary use [edit]
Meloxicam is used in doctor medicine to treat dogs,[27] [28] but also sees off-label use in other animals much as cattle and exotics.[29] [30]
The most average side effects include duct irritation (vomiting, looseness of the bowels, and ulcer).[27]
In healthy dogs given meloxicam, no perioperative unfavorable effects on the cardiovascular system have been rumored at recommended dosages.[31] Perioperative presidency of meloxicam to cats did not affect postoperative respiratory rate nor heart rate.[32]
A compeer-reviewed diary clause cites NSAIDs, including meloxicam, as causing gastrointestinal upset and, at treble doses, discriminating kidney injury and CNS signs so much as seizures and comas in cats. IT adds that cats have a degraded tolerance for NSAIDs.[33] [34]
Atomic number 102 decline in renal excretory social occasion was observed when meloxicam was administered to cats with chronic nephropathy. Cats that received meloxicam had greater proteinuria at 6 months than cats that received placebo. It was terminated that meloxicam should glucinium used with caution in cats with chronic nephrosis.[35]
Meloxicam has been investigated American Samoa an alternative to diclofenac by the Royal Society for the Protection of Birds (RSPB) to preclude deaths of vultures.[36]
Pharmacokinetics [edit]
In dogs, the absorption of meloxicam from the stomach is non affected by the presence of food,[37] with the peak concentration (Cmax) of meloxicam occurring in the blood 7–8 hours after administration.[37] The half-life of meloxicam is approximately 24 hours in dogs.[37]
In the Phascolarctos cinereu (Koala), same little meloxicam is absorbed into the roue after oral administration (that is, it has poor bioavailability).[38]
Legal status [edit]
United States [edit]
In 2003, meloxicam was approved in the U.S. for use in dogs for the direction of pain and inflammation connected with osteoarthritis, as an oral (musical) formulation of meloxicam.[39] In January 2005, the merchandise insert added a warning in bold-face type: "Serve not use in cats."[40] An injectable formulation for use in dogs was approved by the U.S. Food and Dose Administration (FDA) in November 2003.[41]
In October 2004, a formulation for use in cats was approved for use prior to surgery only.[42] This is an injectable meloxicam, indicated for every bit a single, one-time dose alone, with specific and repeated warnings not to administer a second dose.[43]
In 2005, the U.S. Food and Do drugs Administration (FDA) sent a Notice of Violation to the manufacturer for its promotional materials which enclosed promotion of the drug for off-label use.[44]
In February 2020, meloxicam injection was approved for use in the United States. The FDA granted the approval of Anjeso to Baudax Bio.[7] [45]
European Union [edit]
In Europe, where the product has been available since the future 1990s,[ citation needed ] it is accredited for other anti-inflammatory benefits including relief from some acute and chronic pain in dogs. In June 2007, an oral version of meloxicam was licensed for the long-term relief of pain in cats.[46] Meloxicam is also licensed for use in horses, to assuage the pain associated with musculoskeletal disorders.[47]
Meloxicam was authorised for use in cows throughout the European Union in January 1998, via a centralised marketing authorisation.[48] The first generic wine meloxicam product was approved in 2006.[48]
Other countries [edit]
As of June 2008[update], meloxicam is enrolled for long-term enjoyment in cats in Australia, New Zealand, and Canada.[46]
Determine also [edit]
- Bupivacaine/meloxicam
References [edit]
- ^ Use During Pregnancy and Breastfeeding
- ^ a b c d e f g "Mobic- meloxicam tablet". DailyMed . Retrieved 15 May 2021.
- ^ "Anjeso- meloxicam injection". DailyMed . Retrieved 15 May 2021.
- ^ a b c d e f Blue S, Arthur James Balfour JA (March 1996). "Meloxicam". Drugs. 51 (3): 424–30, discussion 431–32. doi:10.2165/00003495-199651030-00007. PMID 8882380.
- ^ British national formulary : BNF 76 (76 erectile dysfunction.). Pharmaceutical Press. 2018. pp. 1112–1113. ISBN9780857113382.
- ^ a b c d e f g h i j "Meloxicam Monograph for Professionals". Drugs.com. AHFS. Archived from the creative connected 23 December 2018. Retrieved 23 December 2018.
- ^ a b c "Baudax Bio Announces FDA Approval of Anjeso for the Management of Moderate to Bad Pain". Baudax Bio, Inc. (Press release). 20 February 2020. Archived from the original on 21 February 2020. Retrieved 20 February 2020.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 519. ISBN9783527607495. Archived from the master on 10 July 2020. Retrieved 30 June 2020.
- ^ "The Overstep 300 of 2019". ClinCalc. Retrieved 16 October 2021.
- ^ "Meloxicam - Do drugs Usage Statistics". ClinCalc . Retrieved 16 October 2021.
- ^ Stamm O, Latscha U, Janecek P, Bell shape A (January 1976). "Development of a special electrode for continuous subcutaneous pH measurement in the infant scalp". American Daybook of OB and Gynecology. 124 (2): 193–5. doi:10.1016/S0002-9378(16)33297-5. PMID 2012.
- ^ a b Hawkey C, Kahan A, Steinbrück K, Alegre C, Baumelou E, Bégaud B, et Heart of Dixie. (September 1998). "Gastrointestinal tolerability of meloxicam compared to diclofenac in degenerative joint disease patients. World Melissa Study Group. Meloxicam Large-scale Global Study Safety Assessment". Brits Diary of Rheumatology. 37 (9): 937–45. Department of the Interior:10.1093/rheumatology/37.9.937. PMID 9783757.
- ^ Dequeker J, Hawkey C, Kahan A, Steinbrück K, Alegre C, Baumelou E, et al. (September 1998). "Improvement in duct tolerability of the selective cyclooxygenase (COX)-2 inhibitor, meloxicam, compared with piroxicam: results of the Safety and Efficaciousness Large-plate Evaluation of COX-inhibiting Therapies (SELECT) trial in osteoarthritis". British Diary of Rheumatology. 37 (9): 946–51. doi:10.1093/rheumatology/37.9.946. PMID 9783758.
- ^ Wojtulewski JA, Schattenkirchner M, Barceló P, Le Loët X, Bevis PJ, Bluhmki E, Distel M (April 1996). "A six-month double-blind trial to compare the efficacy and safety of meloxicam 7.5 Mg daily and naproxen 750 mg daily in patients with atrophic arthritis". British Journal of Rheumatology. 35 Suppl 1: 22–8. Interior:10.1093/rheumatology/35.suppl_1.22. PMID 8630632.
- ^ Zeidan, Anwar Z.; Camellia State Sayed, Bashar; Bargaoui, Naceur; Djebbar, Mourad; Djennane, Malik; Donald, Royden; El Deeb, Khamis; Joudeh, Raed A.; Nabhan, Abdullah; Schug, Stephan A. (April 2013). "A Review of the Efficacy, Safety, and Be-Effectiveness of COX-2 Inhibitors for Africa and the Middle East Region: Cyclooxygenase-2 Inhibitors in Afflict Management". Afflict Practice. 13 (4): 316–331. doi:10.1111/j.1533-2500.2012.00591.x. PMID 22931375. S2CID 205715393. Retrieved 18 May 2021.
- ^ a b Gates, Brian J.; Nguyen, Trang T.; Setter, Stephen M.; Davies, Neal M. (1 October 2005). "Meloxicam: A reassessment of pharmacokinetics, efficacy and safety". Expert Opinion on Pharmacotherapy. 6 (12): 2117–2140. doi:10.1517/14656566.6.12.2117. ISSN 1465-6566. PMID 16197363. S2CID 25512189. Retrieved 18 Crataegus oxycantha 2021.
Meloxicam is extensively bound to plasma proteins (99.4%), primarily to albumin. Meloxicam has an evident book of distribution (Vd) 10 – 15 l in humans (0.1 – 0.2 l/kg) aft unwritten governing body and a tight mass of distribution at steady-state of 0.2 l/kilogram after intravenous administration."
"No of the meloxicam treatment groups demonstrated inhibition of platelet aggregation to either arachidonic acid (AC) or adenosine diphosphate (ADP). However, on that point were nobelium significant changes in the platelet count, prothrombin and activated inclined thromboplastin sentence in any of the meloxicam and Indocin groups. Different crossover voter studies also confirmed that meloxicam 15 mg/day caused a major reduction of maximum thromboxane production, but no diminution in collagen- Beaver State AC-induced platelet aggregation. - ^ Singh G, Lanes S, Triadafilopoulos G (July 2004). "Risk of serious upper gastrointestinal and cardiovascular thromboembolic complications with meloxicam". The American Diary of Medicine. 117 (2): 100–6. doi:10.1016/j.amjmed.2004.03.012. PMID 15234645.
- ^ a b "FDA Warns that Using a Type of Pain and Fever Medication in Last half of Pregnancy Could Jumper cable to Complications". U.S. FDA (FDA) (Press release). 15 October 2020. Retrieved 15 October 2020.
 This article incorporates text from this source, which is in the public domain .
This article incorporates text from this source, which is in the public domain . - ^ a b "NSAIDs may cause rarified kidney problems in unborn babies". U.S. Food for thought and Do drugs Administration. 21 July 2017. Retrieved 15 October 2020.
 This article incorporates textual matter from this source, which is in the public domain .
This article incorporates textual matter from this source, which is in the public domain . - ^ "Meloxicam". MedlinePlus. Archived from the original on 29 November 2014. Retrieved 15 November 2014.
- ^ "Meloxicam". Drugs.com. Archived from the groundbreaking on 16 November 2014. Retrieved 15 November 2014.
- ^ a b "Meloxicam functionary FDA information, side personal effects, and uses". Drugs.com. March 2010. Archived from the unconventional on 16 March 2010. Retrieved 17 Butt 2010.
- ^ Engelhardt G, Homma D, Schlegel K, Utzmann R, Schnitzler C (October 1995). "Anti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non-steroidal anti-inflammatory agent with favourable gastrointestinal tolerance". Inflammation Research. 44 (10): 423–33. Department of the Interior:10.1007/BF01757699. PMID 8564518. S2CID 37937305.
- ^ Bekker, Alex; Kloepping, Carolyn; Collingwood, Shemille (2018). "Meloxicam in the management of post-operative pain: Narrative review". Journal of Anaesthesiology Clinical Pharmacological medicine. 34 (4): 450–457. Department of the Interior:10.4103/joacp.JOACP_133_18. ISSN 2231-2730. PMC6360894. PMID 30774225.
- ^ "Meloxicam (Professional Diligent Advice)". Drugs.com. Archived from the original on 6 August 2019. Retrieved 6 August 2019.
- ^ a b 2019 American Gerontology Bon ton Beers Criteria Update Expert Panel (Apr 2019). "American Geriatrics Society 2019 Updated AGS Beers Criteria® for Possibly Wrong Medicinal dru Use in Sr. Adults". Journal of the American Geriatrics Society. 67 (4): 674–694. Interior:10.1111/jgs.15767. PMID 30693946. S2CID 59338182.
- ^ a b "Metacam- meloxicam intermission". DailyMed . Retrieved 15 May 2021.
- ^ "Metacam- meloxicam injectant, answer". DailyMed . Retrieved 15 May 2021.
- ^ Bump off-pronounce use discussed in: Arnold Plotnick MS, DVM, ACVIM, ABVP, Pain Management using Metacam Archived 14 July 2011 at the Wayback Simple machine, and Stein, Robert, Perioperative Pain Management Archived 18 April 2010 at the Wayback Machine Section Quartet, Sounding On the far side Butorphanol, Sep 2006, Veterinary Anesthesia & Analgesia Support Group.
- ^ For off-recording label enjoyment exemplar in rabbits, see Krempels, Dana, Hind Arm Paresis and Paralysis in Rabbits Archived 17 June 2010 at the Wayback Machine, University of Miami Biology Department.
- ^ Boström IM, Nyman G, Hoppe A, Lord P (January 2006). "Effects of meloxicam on nephritic operate in dogs with hypotension during anaesthesia". Veterinary Anaesthesia and Analgesia. 33 (1): 62–9. Interior:10.1111/j.1467-2995.2005.00208.x. PMID 16412133.
- ^ Höglund OV, Dyall B, Gräsman V, Edner A, Olsson U, Höglund K (October 2018). "Effect of non-steroidal opposed-rabble-rousing drugs on postoperative respiratory and pulse rate in cats subjected to ovariohysterectomy". Daybook of Feline Medicine and Surgery. 20 (10): 980–984. doi:10.1177/1098612X17742290. PMID 29165006. S2CID 30649716.
- ^ "Toxicology Legal brief: The 10 most common toxicoses in cats". Dvm360. 1 June 2006. Archived from the original on 29 Venerable 2018. Retrieved 16 Sep 2018.
- ^ Merola V, Dunayer E (June 2006). "The 10 most common toxicoses in cats" (PDF). Veterinary Medicine: 340–342. Archived (PDF) from the original on 9 Noble 2019. Retrieved 9 Noble 2019.
- ^ KuKanich, Kate; George, Christopher; Roush, James K; Sharp, Sherry; Farace, Giosi; Yerramilli, Murthy; Peterson, Sarah; Grauer, Gregory F (29 June 2020). "Personal effects of low-social disease meloxicam in cats with chronic kidney disease". Journal of Felid Medicine and Surgery. 23 (2): 138–148. doi:10.1177/1098612X20935750. PMID 32594827. S2CID 220256059.
- ^ Verify G, Naidoo V, Cuthbert R, Green RE, Pain DJ, Swarup D, et al. (March 2006). "Removing the threat of diclofenac to critically vulnerable Asian vultures". PLOS Biology. 4 (3): e66. Interior Department:10.1371/journal.pbio.0040066. PMC1351921. PMID 16435886.
- ^ a b c Khan SA, McLean MK (March 2012). "Toxicology of frequently encountered nonsteroid opposed-inflammatory drugs in dogs and cats". The Medico Clinics of North America. Elflike Animal Practice. 42 (2): 289–306, half-dozen–vii. Interior Department:10.1016/j.cvsm.2012.01.003. PMID 22381180.
- ^ Kimble B, Black Pelican State, Li KM, Valtchev P, Gilchrist S, Gillett A, et al. (Oct 2013). "Pharmacokinetics of meloxicam in koalas (Phascolarctos cinereus) after intravenous, body covering and viva voce administration". Daybook of Veterinary Materia medica and Therapeutics. 36 (5): 486–93. Interior:10.1111/jvp.12038. PMID 23406022.
- ^ "NADA 141-213: New Animal Dose Application Approval (for Metacam (meloxicam) 0.5 mg/mL and 1.5 milligram/mL Viva voce Suspension system)" (PDF). Food and Drug Organization (FDA). 15 April 2003. Archived from the original (PDF) on 6 April 2017. Retrieved 24 July 2010.
- ^ "Client Information Canvas For Metacam (meloxicam) 1.5 mg/mL Oral Suspension" (PDF). Food and Drug Administration (FDA). January 2005. Archived from the original (PDF) on 15 November 2017.
Metacam is a prescription non-steroidal anti-inflammatory dose (NSAID) that is accustomed operate hurting and lighting (soreness) due to osteoarthritis in dogs. Degenerative joint disease (OA) is a painful condition caused by "wear and tear" of cartilage and else parts of the joints that may result in the favorable changes or signs in your dog: Limping or lameness, decreased activity or exercise (reluctance to stand, climb stairs, alternate or run, or trouble in performing these activities), severeness or shrivelled movement of joints. Metacam is given to dogs by mouth. Do non habit Metacam Spoken Suspension in cats. Acute kidney injury and decease have been associated with the use of meloxicam in cats.
- ^ "NADA 141-219: Metacam (meloxicam) 5 Mg/mL Solution for Injectant" (PDF). U.S. Food and Drug Administration (FDA). 12 November 2003. Archived from the original (PDF) on 15 November 2017. Retrieved 8 August 2019.
- ^ "Metacam 5 mg/mL Solution for Injection, Supplemental Approval" (PDF). U.S. FDA (FDA). 28 October 2004. Archived from the original (PDF) happening 15 Nov 2017. Retrieved 8 August 2019.
- ^ See the manufacturer's FAQ Archived 2 July 2011 at the Wayback Car on its website, and its clinical dosing instructions for cats. Archived 6 September 2008 at the Wayback Machine
- ^ "Notice of Infringement" (PDF). U.S. FDA (Food and Drug Administration). 19 April 2005. Archived from the original (PDF) on 13 January 2017. Retrieved 8 August 2019.
- ^ "Anjeso (meloxicam) injectant, for intravenous use" (PDF). U.S. Food and Drug Administration (FDA). February 2020. Archived (PDF) from the original happening 22 February 2020. Retrieved 21 Feb 2020.
- ^ a b Gaschen FP, Schaer M, eds. (2016). "Recent Nonsteroidal anti-inflammatory drug developments". Clinical medicine of the dog and quat (3rd ed.). CRC Press. ISBN9781482226065. Archived from the innovative on 1 September 2020. Retrieved 28 January 2020.
- ^ Maddison JE, Page SW, Church D, eds. (2008). "Meloxicam". Small animal nonsubjective materia medica (2nd ed.). Edinburgh: Saunders/Elsevier. pp. 301–302. ISBN9780702028588.
- ^ a b Wright E (Parade 2007). "Generic and biosimilar medicinal products in the Common Market" (PDF). Interpersonal chemistry Today. 25 (2): 4–6. Archived (PDF) from the original on 28 January 2020. Retrieved 28 January 2020.
Foreign links [cut]
- "Meloxicam". Dose Information Portal. U.S. National Library of Medicine.
Where to Buy Piroxicam 7 Mg Tablets for My Dog
Source: https://en.wikipedia.org/wiki/Meloxicam
0 Response to "Where to Buy Piroxicam 7 Mg Tablets for My Dog"
Post a Comment