what process causes surface water to become water vapor
| Water vapor (HiiO) | |
|---|---|
 Invisible water vapor condenses to class | |
| Liquid country | Water |
| Solid state | Ice |
| Properties[1] | |
| Molecular formula | HiiO |
| Tooth mass | eighteen.01528(33) one thousand/mol |
| Melting point | 0.00 °C (273.fifteen 1000)[2] |
| Boiling point | 99.98 °C (373.13 K)[2] |
| specific gas constant | 461.5 J/(kg·K) |
| Estrus of vaporization | ii.27 MJ/kg |
| Heat capacity at 300 K | ane.864 kJ/(kg·One thousand)[3] |
Water vapor, water vapour or aqueous vapor is the gaseous stage of water. Information technology is one state of water within the hydrosphere. Water vapor tin be produced from the evaporation or boiling of liquid water or from the sublimation of ice. Water vapor is transparent, like most constituents of the temper.[four] Under typical atmospheric weather condition, water vapor is continuously generated past evaporation and removed by condensation. It is less dense than near of the other constituents of air and triggers convection currents that can atomic number 82 to clouds.
Being a component of Earth's hydrosphere and hydrologic wheel, it is peculiarly abundant in Earth's atmosphere, where information technology acts as a greenhouse gas and warming feedback, contributing more to total greenhouse effect than non-condensable gases such as carbon dioxide and methane. Utilise of h2o vapor, every bit steam, has been important for cooking, and as a major component in energy production and transport systems since the industrial revolution.
Water vapor is a relatively common atmospheric constituent, nowadays even in the solar temper likewise as every planet in the Solar System and many astronomical objects including natural satellites, comets and even large asteroids. Likewise the detection of extrasolar water vapor would bespeak a similar distribution in other planetary systems. Water vapor is significant in that information technology can be indirect bear witness supporting the presence of extraterrestrial liquid h2o in the case of some planetary mass objects.
Properties [edit]
Evaporation [edit]
Whenever a h2o molecule leaves a surface and diffuses into a surrounding gas, it is said to have evaporated. Each individual water molecule which transitions between a more than associated (liquid) and a less associated (vapor/gas) state does so through the absorption or release of kinetic energy. The aggregate measurement of this kinetic energy transfer is defined as thermal energy and occurs only when there is differential in the temperature of the water molecules. Liquid h2o that becomes water vapor takes a packet of heat with it, in a process called evaporative cooling.[5] The amount of water vapor in the air determines how oft molecules will return to the surface. When a net evaporation occurs, the body of water volition undergo a net cooling directly related to the loss of water.
In the United states of america, the National Conditions Service measures the actual charge per unit of evaporation from a standardized "pan" open up h2o surface outdoors, at various locations nationwide. Others do also effectually the earth. The United states of america data is collected and compiled into an almanac evaporation map.[6] The measurements range from nether xxx to over 120 inches per year. Formulas can be used for calculating the charge per unit of evaporation from a h2o surface such as a swimming pool.[7] [viii] In some countries, the evaporation rate far exceeds the precipitation rate.
Evaporative cooling is restricted by atmospheric weather. Humidity is the corporeality of h2o vapor in the air. The vapor content of air is measured with devices known as hygrometers. The measurements are usually expressed equally specific humidity or per centum relative humidity. The temperatures of the temper and the h2o surface decide the equilibrium vapor pressure; 100% relative humidity occurs when the partial force per unit area of water vapor is equal to the equilibrium vapor pressure level. This condition is ofttimes referred to as complete saturation. Humidity ranges from 0 grams per cubic metre in dry air to xxx grams per cubic metre (0.03 ounce per cubic foot) when the vapor is saturated at 30 °C.[9]


Sublimation [edit]
Sublimation is the process by which water molecules directly exit the surface of ice without first becoming liquid water. Sublimation accounts for the slow mid-wintertime disappearance of ice and snow at temperatures too low to crusade melting. Antarctica shows this effect to a unique degree considering information technology is by far the continent with the lowest rate of precipitation on Earth. As a event, at that place are big areas where millennial layers of snowfall have sublimed, leaving behind whatever not-volatile materials they had contained. This is extremely valuable to certain scientific disciplines, a dramatic example existence the drove of meteorites that are left exposed in unparalleled numbers and excellent states of preservation.
Sublimation is important in the preparation of certain classes of biological specimens for scanning electron microscopy. Typically the specimens are prepared past cryofixation and freeze-fracture, after which the broken surface is freeze-etched, being eroded past exposure to vacuum till information technology shows the required level of item. This technique can display protein molecules, organelle structures and lipid bilayers with very low degrees of distortion.
Condensation [edit]

Clouds, formed past condensed water vapor
Water vapor will only condense onto another surface when that surface is libation than the dew point temperature, or when the water vapor equilibrium in air has been exceeded. When water vapor condenses onto a surface, a cyberspace warming occurs on that surface.[10] The water molecule brings heat energy with it. In turn, the temperature of the temper drops slightly.[11] In the atmosphere, condensation produces clouds, fog and atmospheric precipitation (usually merely when facilitated by deject condensation nuclei). The dew point of an air parcel is the temperature to which it must cool before h2o vapor in the air begins to condense. Condensation in the atmosphere forms deject droplets.
Also, a cyberspace condensation of water vapor occurs on surfaces when the temperature of the surface is at or beneath the dew point temperature of the temper. Deposition is a phase transition carve up from condensation which leads to the direct formation of water ice from water vapor. Frost and snowfall are examples of deposition.
At that place are several mechanisms of cooling by which condensation occurs: i) Straight loss of heat by conduction or radiation. 2) Cooling from the drop in air force per unit area which occurs with uplift of air, besides known equally adiabatic cooling. Air can be lifted by mountains, which deflect the air upward, by convection, and by cold and warm fronts. 3) Advective cooling - cooling due to horizontal movement of air.
Importance and Uses [edit]
- Provides water for plants and animals: Water vapour gets converted to rain and snow that serve as a natural source of water for plants and animals.
- Controls evaporation: Excess water vapor in the air decreases the rate of evaporation.
- Determines climatic conditions: Backlog water vapor in the air produces rain, fog, snow etc. Hence, information technology determines climatic conditions.
Chemical reactions [edit]
A number of chemical reactions accept water as a product. If the reactions take place at temperatures higher than the dew point of the surrounding air the water will be formed as vapor and increase the local humidity, if below the dew point local condensation will occur. Typical reactions that issue in water formation are the burning of hydrogen or hydrocarbons in air or other oxygen containing gas mixtures, or as a result of reactions with oxidizers.
In a similar fashion other chemical or concrete reactions can have place in the presence of water vapor resulting in new chemicals forming such equally rust on iron or steel, polymerization occurring (certain polyurethane foams and cyanoacrylate glues cure with exposure to atmospheric humidity) or forms irresolute such equally where anhydrous chemicals may absorb plenty vapor to form a crystalline structure or alter an existing one, sometimes resulting in feature colour changes that can be used for measurement.
Measurement [edit]
Measuring the quantity of water vapor in a medium can exist done direct or remotely with varying degrees of accuracy. Remote methods such electromagnetic absorption are possible from satellites above planetary atmospheres. Direct methods may utilise electronic transducers, moistened thermometers or hygroscopic materials measuring changes in physical properties or dimensions.
| medium | temperature range (degC) | measurement uncertainty | typical measurement frequency | system price | notes | |
|---|---|---|---|---|---|---|
| Sling psychrometer | air | −ten to l | low to moderate | hourly | depression | |
| Satellite-based spectroscopy | air | −eighty to 60 | low | very high | ||
| Capacitive sensor | air/gases | −40 to 50 | moderate | 2 to 0.05 Hz | medium | prone to becoming saturated/contaminated over time |
| Warmed capacitive sensor | air/gases | −15 to l | moderate to low | ii to 0.05 Hz (temp dependant) | medium to high | prone to condign saturated/contaminated over time |
| Resistive sensor | air/gases | −x to 50 | moderate | lx seconds | medium | prone to contamination |
| Lithium chloride dewcell | air | −xxx to fifty | moderate | continuous | medium | see dewcell |
| Cobalt(2) chloride | air/gases | 0 to 50 | high | v minutes | very low | often used in Humidity indicator menu |
| Assimilation spectroscopy | air/gases | moderate | high | |||
| Aluminum oxide | air/gases | moderate | medium | meet Moisture analysis | ||
| Silicon oxide | air/gases | moderate | medium | encounter Moisture assay | ||
| Piezoelectric sorption | air/gases | moderate | medium | meet Wet analysis | ||
| Electrolytic | air/gases | moderate | medium | see Wet assay | ||
| Hair tension | air | 0 to xl | high | continuous | low to medium | Afflicted past temperature. Adversely affected past prolonged loftier concentrations |
| Nephelometer | air/other gases | depression | very high | |||
| Goldbeater'due south skin (Cow Peritoneum) | air | −xx to xxx | moderate (with corrections) | slow, slower at lower temperatures | low | ref:WMO Guide to Meteorological Instruments and Methods of Observation No. 8 2006, (pages 1.12–1) |
| Lyman-alpha | high frequency | loftier | http://amsglossary.allenpress.com/glossary/search?id=lyman-alpha-hygrometer1 Requires frequent calibration | |||
| Gravimetric Hygrometer | very low | very loftier | often chosen primary source, national independent standards developed in US,Great britain,EU & Nippon | |||
| medium | temperature range (degC) | measurement uncertainty | typical measurement frequency | system cost | notes |
Impact on air density [edit]
Water vapor is lighter or less dense than dry air.[12] [13] At equivalent temperatures it is buoyant with respect to dry out air, whereby the density of dry out air at standard temperature and force per unit area (273.fifteen Thou, 101.325 kPa) is i.27 grand/L and water vapor at standard temperature has a vapor pressure of 0.vi kPa and the much lower density of 0.0048 g/L.
Calculations [edit]

H2o vapor and dry air density calculations at 0 °C:
- The tooth mass of water is xviii.02 g/mol, equally calculated from the sum of the atomic masses of its elective atoms.
- The average molar mass of air (approx. 78% nitrogen, Due north2; 21% oxygen, Otwo; i% other gases) is 28.57 grand/mol at standard temperature and pressure (STP).
- Obeying Avogadro's Law and the ideal gas law, moist air will have a lower density than dry air. At max. saturation (i. eastward. rel. humidity = 100% at 0 °C) the density volition go down to 28.51 g/mol.
- STP conditions imply a temperature of 0 °C, at which the ability of h2o to get vapor is very restricted. Its concentration in air is very low at 0 °C. The red line on the chart to the right is the maximum concentration of water vapor expected for a given temperature. The water vapor concentration increases significantly as the temperature rises, approaching 100% (steam, pure h2o vapor) at 100 °C. However the difference in densities betwixt air and h2o vapor would nevertheless exist (0.598 vs. 1.27 k/fifty).
At equal temperatures [edit]
At the aforementioned temperature, a cavalcade of dry air will be denser or heavier than a cavalcade of air containing whatever h2o vapor, the molar mass of diatomic nitrogen and diatomic oxygen both existence greater than the molar mass of water. Thus, whatever volume of dry air volition sink if placed in a larger volume of moist air. Also, a volume of moist air volition rise or exist buoyant if placed in a larger region of dry out air. As the temperature rises the proportion of water vapor in the air increases, and its buoyancy will increment. The increment in buoyancy tin accept a significant atmospheric bear upon, giving rise to powerful, wet rich, upward air currents when the air temperature and sea temperature reaches 25 °C or above. This phenomenon provides a pregnant driving force for cyclonic and anticyclonic weather systems (typhoons and hurricanes).
Respiration and breathing [edit]
Water vapor is a by-production of respiration in plants and animals. Its contribution to the pressure, increases as its concentration increases. Its fractional pressure contribution to air pressure level increases, lowering the partial force per unit area contribution of the other atmospheric gases (Dalton's Police). The total air force per unit area must remain constant. The presence of h2o vapor in the air naturally dilutes or displaces the other air components as its concentration increases.
This can have an consequence on respiration. In very warm air (35 °C) the proportion of water vapor is big enough to give rise to the stuffiness that can exist experienced in humid jungle conditions or in poorly ventilated buildings.
Lifting gas [edit]
Water vapor has lower density than that of air and is therefore buoyant in air but has lower vapor pressure than that of air. When water vapor is used as a lifting gas past a thermal airship the water vapor is heated to class steam and then that its vapor pressure is greater than the surrounding air force per unit area in order to maintain the shape of a theoretical "steam balloon", which yields approximately threescore% the lift of helium and twice that of hot air.[fourteen]
General discussion [edit]
The amount of water vapor in an atmosphere is constrained by the restrictions of partial pressures and temperature. Dew bespeak temperature and relative humidity human activity as guidelines for the process of h2o vapor in the water cycle. Energy input, such as sunlight, tin can trigger more evaporation on an sea surface or more sublimation on a chunk of water ice on tiptop of a mountain. The balance between condensation and evaporation gives the quantity called vapor partial pressure.
The maximum fractional pressure (saturation pressure) of h2o vapor in air varies with temperature of the air and h2o vapor mixture. A multifariousness of empirical formulas exist for this quantity; the almost used reference formula is the Goff-Gratch equation for the SVP over liquid water below zero degrees Celsius:
where T, temperature of the moist air, is given in units of kelvin, and p is given in units of millibars (hectopascals).
The formula is valid from virtually −50 to 102 °C; nevertheless there are a very limited number of measurements of the vapor force per unit area of water over supercooled liquid water. There are a number of other formulae which tin be used.[15]
Under certain conditions, such equally when the boiling temperature of water is reached, a cyberspace evaporation will e'er occur during standard atmospheric atmospheric condition regardless of the percent of relative humidity. This immediate process volition dispel massive amounts of water vapor into a libation atmosphere.
Exhaled air is almost fully at equilibrium with water vapor at the trunk temperature. In the cold air the exhaled vapor quickly condenses, thus showing up as a fog or mist of h2o droplets and as condensation or frost on surfaces. Forcibly condensing these water droplets from exhaled jiff is the basis of exhaled breath condensate, an evolving medical diagnostic test.
Decision-making water vapor in air is a primal concern in the heating, ventilating, and ac (HVAC) manufacture. Thermal comfort depends on the moist air conditions. Non-human condolement situations are chosen refrigeration, and too are affected past water vapor. For example, many food stores, similar supermarkets, utilize open chiller cabinets, or food cases, which can significantly lower the water vapor pressure level (lowering humidity). This practice delivers several benefits besides as problems.
In Earth'south temper [edit]
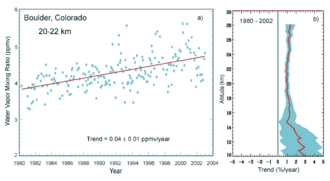
Evidence for increasing amounts of stratospheric water vapor over time in Bedrock, Colorado.
Gaseous water represents a pocket-sized simply environmentally significant constituent of the atmosphere. The percentage of h2o vapor in surface air varies from 0.01% at -42 °C (-44 °F)[16] to 4.24% when the dew point is xxx °C (86 °F).[17] Over 99% of atmospheric water is in the form of vapour, rather than liquid water or ice,[18] and approximately 99.13% of the h2o vapour is independent in the troposphere. The condensation of water vapor to the liquid or ice phase is responsible for clouds, rain, snow, and other precipitation, all of which count among the well-nigh pregnant elements of what we experience as weather. Less obviously, the latent rut of vaporization, which is released to the atmosphere whenever condensation occurs, is ane of the virtually important terms in the atmospheric free energy budget on both local and global scales. For example, latent heat release in atmospheric convection is directly responsible for powering destructive storms such equally tropical cyclones and severe thunderstorms. Water vapor is an important greenhouse gas[19] [20] owing to the presence of the hydroxyl bond which strongly absorbs in the infra-red.
Water vapor is the "working medium" of the atmospheric thermodynamic engine which transforms heat energy from sun irradiation into mechanical energy in the form of winds. Transforming thermal energy into mechanical energy requires an upper and a lower temperature level, too equally a working medium which shuttles forth and back between both. The upper temperature level is given by the soil or water surface of the earth, which absorbs the incoming sunday radiations and warms upwardly, evaporating h2o. The moist and warm air at the footing is lighter than its environment and rises upward to the upper limit of the troposphere. At that place the water molecules radiate their thermal energy into outer space, cooling down the surrounding air. The upper temper constitutes the lower temperature level of the atmospheric thermodynamic engine. The water vapor in the now cold air condenses out and falls downwardly to the ground in the form of rain or snow. The now heavier cold and dry air sinks downwards to ground likewise; the atmospheric thermodynamic engine thus establishes a vertical convection, which transports heat from the footing into the upper temper, where the water molecules can radiate information technology to outer space. Due to the earth'due south rotation and the resulting Coriolis forces, this vertical atmospheric convection is also converted into a horizontal convection, in the class of cyclones and anticyclones, which send the water evaporated over the oceans into the interior of the continents, enabling vegetation to grow.[21]
Water in Earth'southward temper is not merely below its humid betoken (100 °C), but at distance it goes below its freezing point (0 °C), due to water'south highly polar attraction. When combined with its quantity, water vapor then has a relevant dew point and frost point, unlike e. thousand., carbon dioxide and methane. Water vapor thus has a scale height a fraction of that of the bulk temper,[22] [23] [24] equally the water condenses and exits, primarily in the troposphere, the lowest layer of the temper.[25] Carbon dioxide (COtwo) and methane, beingness well-mixed in the atmosphere, tend to rise above water vapour. The absorption and emission of both compounds contribute to Earth's emission to space, and thus the planetary greenhouse effect.[23] [26] [27] This greenhouse forcing is directly observable, via distinct spectral features versus h2o vapor, and observed to exist rise with rising CO2 levels.[28] Conversely, adding h2o vapor at loftier altitudes has a asymmetric bear upon, which is why jet traffic[29] [xxx] [31] has a disproportionately high warming effect. Oxidation of methane is likewise a major source of water vapour in the stratosphere,[32] and adds nigh fifteen% to methane's global warming effect.[33]
In the absenteeism of other greenhouse gases, Earth's water vapor would condense to the surface;[34] [35] [36] this has likely happened, possibly more than once. Scientists thus distinguish betwixt non-condensable (driving) and condensable (driven) greenhouse gases, i.e., the above water vapor feedback.[37] [20] [19]
Fog and clouds class through condensation around cloud condensation nuclei. In the absence of nuclei, condensation will only occur at much lower temperatures. Under persistent condensation or degradation, cloud droplets or snowflakes course, which precipitate when they reach a disquisitional mass.
Atmospheric concentration of h2o vapour is highly variable between locations and times, from 10 ppmv in the coldest air to 5% (l 000 ppmv) in boiling tropical air,[38] and can be measured with a combination of country observations, weather balloons and satellites.[39] The water content of the atmosphere as a whole is constantly depleted past precipitation. At the same time it is constantly replenished past evaporation, most prominently from oceans, lakes, rivers, and moist earth. Other sources of atmospheric water include combustion, respiration, volcanic eruptions, the transpiration of plants, and various other biological and geological processes. At any given fourth dimension there is virtually one.29 ten 1016 litres (3.four ten 1015 gal.) of h2o in the atmosphere. The atmosphere holds i part in 2500 of the fresh water, and 1 part in 100,000 of the full h2o on World.[40] The mean global content of water vapor in the temper is roughly sufficient to cover the surface of the planet with a layer of liquid water most 25 mm deep.[41] [42] [43] The mean annual precipitation for the planet is about ane metre, a comparison which implies a rapid turnover of water in the air – on average, the residence fourth dimension of a water molecule in the troposphere is about 9 to 10 days.[43]
Global mean h2o vapour is about 0.25% of the atmosphere by mass and also varies seasonally, in terms of contribution to atmospheric pressure between two.62 hPa in July and 2.33 hPa in December.[44] IPCC AR6 expresses medium conviction in increase of full water vapour at most ane-2% per decade;[45] information technology is expected to increment by around 7% per °C of warming.[41]
Episodes of surface geothermal action, such as volcanic eruptions and geysers, release variable amounts of water vapor into the atmosphere. Such eruptions may exist big in human terms, and major explosive eruptions may inject exceptionally large masses of water uncommonly high into the atmosphere, only as a percent of total atmospheric water, the role of such processes is niggling. The relative concentrations of the various gases emitted past volcanoes varies considerably according to the site and co-ordinate to the item event at any one site. However, water vapor is consistently the commonest volcanic gas; as a dominion, information technology comprises more than than 60% of total emissions during a subaerial eruption.[46]
Atmospheric h2o vapor content is expressed using various measures. These include vapor pressure, specific humidity, mixing ratio, dew bespeak temperature, and relative humidity.
Radar and satellite imaging [edit]
These maps show the average amount of water vapor in a cavalcade of atmosphere in a given month.(click for more detail)

MODIS/Terra global hateful atmospheric water vapor in atm-cm (centimeters of water in an atmospheric column if it condensed)
Because water molecules blot microwaves and other radio moving ridge frequencies, water in the temper attenuates radar signals.[47] In addition, atmospheric water will reflect and refract signals to an extent that depends on whether it is vapor, liquid or solid.
Mostly, radar signals lose strength progressively the farther they travel through the troposphere. Unlike frequencies attenuate at unlike rates, such that some components of air are opaque to some frequencies and transparent to others. Radio waves used for broadcasting and other communication experience the aforementioned effect.
Water vapor reflects radar to a lesser extent than practise water's other two phases. In the form of drops and ice crystals, water acts as a prism, which it does not exercise as an individual molecule; withal, the existence of water vapor in the atmosphere causes the atmosphere to act as a giant prism.[48]
A comparison of GOES-12 satellite images shows the distribution of atmospheric water vapor relative to the oceans, clouds and continents of the Globe. Vapor surrounds the planet only is unevenly distributed. The paradigm loop on the correct shows monthly average of water vapor content with the units are given in centimeters, which is the precipitable water or equivalent amount of water that could be produced if all the water vapor in the cavalcade were to condense. The lowest amounts of water vapor (0 centimeters) appear in xanthous, and the highest amounts (six centimeters) appear in dark blue. Areas of missing data appear in shades of greyness. The maps are based on data collected by the Moderate Resolution Imaging Spectroradiometer (MODIS) sensor on NASA'due south Aqua satellite. The most noticeable pattern in the time series is the influence of seasonal temperature changes and incoming sunlight on water vapor. In the tropics, a band of extremely humid air wobbles due north and south of the equator equally the seasons alter. This band of humidity is role of the Intertropical Convergence Zone, where the easterly merchandise winds from each hemisphere converge and produce near-daily thunderstorms and clouds. Farther from the equator, water vapor concentrations are high in the hemisphere experiencing summer and low in the one experiencing winter. Some other blueprint that shows up in the fourth dimension series is that h2o vapor amounts over land areas decrease more in wintertime months than adjacent ocean areas do. This is largely because air temperatures over country driblet more than in the winter than temperatures over the sea. Water vapor condenses more chop-chop in colder air.[49]
Every bit water vapor absorbs light in the visible spectral range, its absorption tin be used in spectroscopic applications (such equally DOAS) to make up one's mind the amount of h2o vapor in the atmosphere. This is washed operationally, e.g. from the GOME spectrometers on ERS and MetOp.[50] The weaker water vapor absorption lines in the blue spectral range and farther into the UV upward to its dissociation limit around 243 nm are more often than not based on quantum mechanical calculations[51] and are just partly confirmed by experiments.[52]
Lightning generation [edit]
Water vapor plays a key role in lightning production in the atmosphere. From deject physics, usually clouds are the real generators of static charge as found in Globe'southward temper. The ability of clouds to hold massive amounts of electrical free energy is directly related to the amount of water vapor nowadays in the local organisation.
The amount of h2o vapor directly controls the permittivity of the air. During times of low humidity, static belch is quick and like shooting fish in a barrel. During times of higher humidity, fewer static discharges occur. Permittivity and capacitance work hand in hand to produce the megawatt outputs of lightning.[53]
After a cloud, for instance, has started its manner to becoming a lightning generator, atmospheric water vapor acts as a substance (or insulator) that decreases the ability of the cloud to discharge its electrical energy. Over a certain amount of time, if the deject continues to generate and shop more static electricity, the barrier that was created by the atmospheric water vapor will ultimately pause down from the stored electrical potential energy.[54] This energy will be released to a local oppositely charged region, in the form of lightning. The strength of each belch is directly related to the atmospheric permittivity, capacitance, and the source's charge generating power.[55]
[edit]
Water vapor is common in the Solar System and past extension, other planetary systems. Its signature has been detected in the atmospheres of the Sun, occurring in sunspots. The presence of water vapor has been detected in the atmospheres of all vii extraterrestrial planets in the solar arrangement, the Earth's Moon,[56] and the moons of other planets,[ which? ] although typically in only trace amounts.


Artist's analogy of the signatures of water in exoplanet atmospheres detectable by instruments such every bit the Hubble Space Telescope.[58]
Geological formations such as cryogeysers are thought to be on the surface of several icy moons ejecting water vapor due to tidal heating and may point the presence of substantial quantities of subsurface water. Plumes of h2o vapor take been detected on Jupiter's moon Europa and are like to plumes of water vapor detected on Saturn's moon Enceladus.[57] Traces of water vapor have also been detected in the stratosphere of Titan.[59] Water vapor has been constitute to exist a major constituent of the atmosphere of dwarf planet, Ceres, largest object in the asteroid belt[lx] The detection was made past using the far-infrared abilities of the Herschel Space Observatory.[61] The finding is unexpected because comets, non asteroids, are typically considered to "sprout jets and plumes." According to i of the scientists, "The lines are condign more and more blurred between comets and asteroids."[61] Scientists studying Mars hypothesize that if water moves about the planet, it does and then as vapor.[62]
The luminescence of comet tails comes largely from h2o vapor. On approach to the Lord's day, the ice many comets bear sublimes to vapor. Knowing a comet's distance from the dominicus, astronomers may deduce the comet'due south water content from its brilliance.[63]
Water vapor has also been confirmed outside the Solar System. Spectroscopic analysis of Hd 209458 b, an extrasolar planet in the constellation Pegasus, provides the commencement evidence of atmospheric water vapor across the Solar System. A star called CW Leonis was institute to have a ring of vast quantities of h2o vapor circling the aging, massive star. A NASA satellite designed to written report chemicals in interstellar gas clouds, made the discovery with an onboard spectrometer. Most probable, "the water vapor was vaporized from the surfaces of orbiting comets."[64] Other exoplanets with testify of water vapor include HAT-P-11b and K2-18b.[65] [66]
Come across also [edit]
- Air density
- Atmospheric river
- Humid point
- Condensation in aerosol dynamics
- Deposition
- Earth's atmosphere
- Eddy covariance
- Equation of state
- Evaporative cooler
- Fog
- Frost
- Gas laws
- Gibbs free energy
- Gibbs phase rule
- Greenhouse gas
- Heat capacity
- Heat of vaporization
- Humidity
- Hygrometer
- Ideal gas
- Kinetic theory of gases
- Latent heat
- Latent heat flux
- Microwave radiometer
- Stage of affair
- Saturation vapor density
- Steam
- Sublimation
- Superheating
- Supersaturation
- Thermodynamics
- Troposphere
- Vapor pressure
References [edit]
- ^ Lide (1992)
- ^ a b SODDI Vienna Standard Mean Bounding main Water (VSMOW), used for calibration, melts at 273.1500089(ten) K (0.000089(10) °C, and boils at 373.1339 [Kelvin|1000} (99.9839 °C)
- ^ "Water Vapor – Specific Estrus". Retrieved May fifteen, 2012.
- ^ "What is Water Vapor?". Retrieved August 28, 2012.
- ^ Schroeder (2000), p. 36
- ^ https://web.archive.org/web/20080412215652/http://www.grow.arizona.edu/Abound--GrowResources.php?ResourceId=208. Archived from the original on April 12, 2008. Retrieved April vii, 2008.
- ^ "pond, pool, calculation, evaporation, water, thermal, temperature, humidity, vapor, excel". Retrieved February 26, 2016.
- ^ "Summary of Results of all Puddle Evaporation Charge per unit Studies". R. L. Martin & Associates. Archived from the original on March 24, 2008.
- ^ "climate - meteorology". Encyclopædia Britannica . Retrieved February 26, 2016.
- ^ Held, Isaac M.; Soden, Brian J. (November 2000). "Watervaporfeedback Andglobalwarming". Annual Review of Energy and the Environment. 25 (i): 441–475. doi:10.1146/annurev.energy.25.ane.441. ISSN 1056-3466.
- ^ Schroeder (2000), p. nineteen
- ^ Williams, Jack (August 5, 2013). "Why dry air is heavier than humid air". The Washington Mail service . Retrieved December 28, 2014.
- ^ "Humidity 101". World H2o rescue Foundation. Archived from the original on April 16, 2013. Retrieved December 28, 2014.
- ^ Goodey, Thomas J. "Steam Balloons and Steam Airships". Retrieved August 26, 2010.
- ^ "Water Vapor Pressure Formulations". Retrieved February 26, 2016.
- ^ McElroy (2002), p. 34, Fig. 4.3a
- ^ McElroy (2002), p. 36 example 4.1
- ^ "Atmospheric H2o Vapor". Remote Sensing Systems . Retrieved August 22, 2021.
- ^ a b Lacis, A. et al., The role of long-lived greenhouse gases as chief LW control knob that governs the global surface temperature for past and time to come climatic change, Tellus B, vol. 65 p. 19734, 2013
- ^ a b "Properties". American Chemical Society . Retrieved February 26, 2016.
- ^ https://web.stanford.edu/~ajlucas/The%20Atmosphere%20as%20a%20Heat%20Engine.pdf [ dead link ]
- ^ Bruce L. Gary. "Ch#5". Retrieved Feb 26, 2016.
- ^ a b "The Carbon Dioxide Greenhouse Upshot". Retrieved February 26, 2016.
- ^ Weaver & Ramanathan (1995)
- ^ Norris, G. (December 2, 2013). "Icy Surprise". Aviation Calendar week & Space Technology. 175 (41): xxx.
22,000 ft., which is considered the upper limit for clouds containing supercooled liquid water
- ^ "Climate scientists confirm elusive tropospheric hot spot". ARC Centre of Excellence for Climate System Science. May xiv, 2015. Archived from the original on April 4, 2019. Retrieved May 17, 2015.
- ^ Sherwood, S; Nishant, North (May 11, 2015). "Atmospheric changes through 2012 as shown past iteratively homogenized radiosonde temperature and wind data (IUKv2)". Environmental Research Messages. ten (5): 054007. Bibcode:2015ERL....10e4007S. doi:10.1088/1748-9326/10/5/054007.
- ^ Feldman, D (February 25, 2015). "Observational decision of surface radiative forcing by CO2 from 2000 to 2010". Nature. 519 (7543): 339–343. Bibcode:2015Natur.519..339F. doi:ten.1038/nature14240. PMID 25731165. S2CID 2137527.
- ^ Messer, A. "Jet contrails alter boilerplate daily temperature range". Retrieved May 17, 2015.
- ^ Danahy, A. "Jets' contrails contribute to heat-trapping high-level clouds". Retrieved May 17, 2015.
- ^ Ryan, A; Mackenzie, A; et al. (September 2012). "Globe War II contrails: a case written report of aviation-induced cloudiness". International Periodical of Climatology. 32 (eleven): 1745–1753. Bibcode:2012IJCli..32.1745R. doi:x.1002/joc.2392.
- ^ Noël, Stefan; Weigel, Katja; et al. (2017). "Water Vapour and Methyl hydride Coupling in the Stratosphere observed with SCIAMACHY Solar Occultation Measurements" (PDF). Atmospheric Chemistry and Physics (18): 4463–4476. doi:10.5194/acp-18-4463-2018. Retrieved August 22, 2021.
- ^ Myhre, Gunnar; et al. (January 9, 2007). "Radiative forcing due to stratospheric h2o vapour from CH4 oxidation". Geophysical Inquiry Messages. 34 (1). Bibcode:2007GeoRL..34.1807M. doi:10.1029/2006GL027472.
- ^ Vogt et al. (2010): "The equilibrium temperature of the Earth is 255 K, well-below the freezing bespeak of water, just because of its atmosphere, the greenhouse issue warms the surface"
- ^ What is the maximum and minimum distance for the Earth that is uniform with life?
- ^ "for the Globe, the albedo is 0.306 and the distance is one.000 AU, then the expected temperature is 254 K or -19 C – significantly beneath the freezing bespeak of water!"
- ^ de Pater, I., Lissauer, J., Planetary Sciences, Cambridge Academy Press, 2007
- ^ Wallace, John M. and Peter V. Hobbs. Atmospheric Science: An Introductory Survey Archived 2018-07-28 at the Wayback Automobile. Elsevier. Second Edition, 2006. ISBN 978-0-12-732951-ii. Page viii.
- ^ Li, Zhenhong; Muller, Jan-Peter; Cantankerous, Paul (October 29, 2003). "Comparing of precipitable h2o vapor derived from radiosonde, GPS, and Moderate-Resolution Imaging Spectroradiometer measurements". Journal of Geophysical Enquiry: Atmospheres. 108 (20): 4651. Bibcode:2003JGRD..108.4651L. doi:10.1029/2003JD003372.
- ^ Gleick, P. H. (1996). "H2o Resources". In Schneider, S. H. (ed.). Encyclopedia of Climate and Weather condition. New York: Oxford University Press. pp. 817–823.
Vol. 2
- ^ a b Forsythe, John; Haar2, Thomas H; Cronk, Heather (May 21, 2014). "Observed Global and Regional Variation in Globe's H2o Vapor: Focus on the Weather-Climate Interface" (PDF) . Retrieved August 22, 2021.
- ^ International Satellite Deject Climatology Project (2010). "21-Year Deviations and Anomalies of Region Monthly Mean From Total Menstruation Mean Over Global Total Column H2o Vapor (cm)". Retrieved August 22, 2021.
- ^ a b Mockler, SB (December 1995). "Water vapor in the climate system". AGU Special Report . Retrieved August 22, 2021.
- ^ Trenberth, Kevin E; Smith, Lesley (March 15, 2005). "The Mass of the Atmosphere: A Constraint on Global Analyses". Journal of Climate. 18 (vi): 864–875. Bibcode:2005JCli...eighteen..864T. doi:10.1175/JCLI-3299.ane. Retrieved August 22, 2021.
- ^ Gulev, S. K., P. W. Thorne, J. Ahn, F. J. Dentener, C. M. Domingues, S. Gerland, D. Gong, D. South. Kaufman, H. C. Nnamchi, J. Quaas, J. A. Rivera, S. Sathyendranath, S. L. Smith, B. Trewin, K. von Shuckmann, R. S. Vose (2021). "2.iii.one.3.3 Total column water vapour". In Masson-Delmotte, Five; P, Zhai (eds.). Irresolute Country of the Climate Organisation. Climate Change 2021: The Physical Science Footing. Contribution of Working Group I to the Sixth Assessment Study of the Intergovernmental Panel on Climate Modify (Report). Cambridge University Press. pp. 52–3. Retrieved Baronial 22, 2021.
{{cite report}}: CS1 maint: uses authors parameter (link) - ^ Sigurdsson & Houghton (2000)
- ^ Skolnik (1990), p. 23.five
- ^ Skolnik (1990), pp. 2.44–2.54
- ^ "Water Vapor". Global Maps. July 31, 2018. Retrieved February 26, 2016.
- ^ Loyola, Diego. "GOME-2/MetOp-A at DLR". atmos.eoc.dlr.de . Retrieved October nineteen, 2017.
- ^ Tennyson, Jonathan (2014). "Vibration–rotation transition dipoles from get-go principles". Journal of Molecular Spectroscopy. 298: 1–half-dozen. Bibcode:2014JMoSp.298....1T. doi:10.1016/j.jms.2014.01.012.
- ^ Tennyson, J., Bernath, P.F., Dark-brown, 50.R., Campargue, A., Carleer, 1000.R., Csa´sza´r, A.Grand., Daumont, L., Gamache, R.R., es, J. T. H., Naumenko, O.5., Polyansky, O.Fifty., Rothmam, L.S., Vandaele, A.C., Zobov, Northward.F., Al Derzi, A.R., F´abri, C., Fazliev, A.Z., rtenbacher, T.F., Gordon, I.E., Lodi, L., and Mizus, I.I. (2013). "IUPAC critical evaluation of the rotational-vibrational spectra of 1440 h2o vapor. Part Three". Concrete Chemistry Chemical Physics. xv (37): 15 371–15 381. Bibcode:2013PCCP...1515371T. doi:10.1039/C3CP50968K. PMID 23928555.
{{cite journal}}: CS1 maint: multiple names: authors listing (link) - ^ Shadowitz (1975), pp. 165–171
- ^ Shadowitz (1975), pp. 172–173, 182, 414–416
- ^ Shadowitz (1975), p. 172
- ^ Sridharan et al. (2010), p. 947
- ^ a b Melt, Jia-Rui C.; Gutro, Rob; Brown, Dwayne; Harrington, J.D.; Fohn, Joe (December 12, 2013). "Hubble Sees Evidence of Water Vapor at Jupiter Moon". NASA . Retrieved December 12, 2013.
- ^ "Hubble traces faint signatures of water in exoplanet atmospheres (creative person's analogy)". ESA/Hubble Press Release . Retrieved December v, 2013.
- ^ Cottini et al. (2012)
- ^ Küppers et al. (2014)
- ^ a b Harrington, J.D. (January 22, 2014). "Herschel Telescope Detects Water on Dwarf Planet – Release xiv-021". NASA . Retrieved January 22, 2014.
- ^ Jakosky, Bruce, et al. "H2o on Mars", April 2004, Physics Today, p. 71.
- ^ Beefcake of a Comet
- ^ Lloyd, Robin. "Water Vapor, Possible Comets, Establish Orbiting Star", xi July 2001, Space.com. Retrieved December 15, 2006.
- ^ Clavin, Whitney; Chou, Felicia; Weaver, Donna; Villard; Johnson, Michele (September 24, 2014). "NASA Telescopes Notice Clear Skies and H2o Vapor on Exoplanet". NASA . Retrieved September 24, 2014.
- ^ Tsiaras, Angelos; et al. (September xi, 2019). "H2o vapour in the atmosphere of the habitable-zone eight-Earth-mass planet K2-xviii b". Nature Astronomy. iii (12): 1086–1091. arXiv:1909.05218. Bibcode:2019NatAs.tmp..451T. doi:10.1038/s41550-019-0878-nine. S2CID 202558393.
Bibliography [edit]
- Cottini, Five.; Nixon, C. A.; Jennings, D. E.; Anderson, C. Chiliad.; Gorius, Due north.; Bjoraker, Grand.L.; Coustenis, A.; Teanby, North. A.; Achterberg, R. K.; Bézard, B.; de Kok, R.; Lellouch, Due east.; Irwin, P. G. J.; Flasar, F. M.; Bampasidis, Chiliad. (2012). "Water vapor in Titan's stratosphere from Cassini CIRS far-infrared spectra". Icarus. 220 (two): 855–862. Bibcode:2012Icar..220..855C. doi:10.1016/j.icarus.2012.06.014. hdl:2060/20140010836.
- Küppers, Michael; O'Rourke, Laurence; Bockelée-Morvan, Dominique; Zakharov, Vladimir; Lee, Seungwon; von Allmen, Paul; Carry, Benoît; Teyssier, David; Marston, Anthony; Müller, Thomas; Crovisier, Jacques; Barucci, M. Antonietta; Moreno, Raphael (2014). "Localized sources of water vapour on the dwarf planet (1) Ceres". Nature. 505 (7484): 525–527. Bibcode:2014Natur.505..525K. doi:10.1038/nature12918. PMID 24451541. S2CID 4448395.
- Lide, David (1992). CRC Handbook of Chemistry and Physics (73rd ed.). CRC Press.
- McElroy, Michael B. (2002). The Atmospheric Environment. Princeton Academy Printing.
- Schroeder, David (2000). Thermal Physics. Addison Wesley Longman.
- Shadowitz, Albert (1975). The Electromagnetic Field. McGraw-Hill.
- Sigurdsson, Haraldur; Houghton, B. F. (2000). Encyclopedia of Volcanoes. San Diego, CA: Academic Printing. ISBN9780126431407.
- Skolnik, Merrill (1990). Radar Handbook (2nd ed.). McGraw-Hill.
- Sridharan, R.; Ahmed, Southward. M.; Dasa, Tirtha Pratim; Sreelathaa, P.; Pradeepkumara, P.; Naika, Neha; Supriya, Gogulapati (2010). "'Directly' show for h2o (H2O) in the sunlit lunar ambience from CHACE on MIP of Chandrayaan I". Planetary and Space Scientific discipline. 58 (vi): 947–950. Bibcode:2010P&SS...58..947S. doi:10.1016/j.pss.2010.02.013.
- Vogt, Steven S.; Butler, R. Paul; Rivera, E. J.; Haghighipour, North.; Henry, Gregory W.; Williamson, Michael H. (2010). "The Lick-Carnegie Exoplanet Survey: a 3.1 One thousand ⊕ planet in the habitable zone of the nearby M3V star Gliese 581" (PDF typhoon). The Astrophysical Journal. 723 (i): 954–965. arXiv:1009.5733. Bibcode:2010ApJ...723..954V. doi:10.1088/0004-637X/723/1/954. S2CID 3163906.
- Weaver, C. P.; Ramanathan, V. (1995). "Deductions from a uncomplicated climate model: factors governing surface temperature and atmospheric thermal construction" (PDF). Periodical of Geophysical Research. 100 (D6): 11585–11591. Bibcode:1995JGR...10011585W. doi:ten.1029/95jd00770.
External links [edit]
- National Science Digital Library – H2o Vapor
- Calculate the condensation of your exhaled breath
- Water Vapor Myths: A Brief Tutorial
- AGU Water Vapor in the Climate Organisation – 1995
- Gratis Windows Program, Water Vapor Pressure level Units Conversion Reckoner – PhyMetrix
Source: https://en.wikipedia.org/wiki/Water_vapor

0 Response to "what process causes surface water to become water vapor"
Post a Comment